Explain the structure of Graphite.
4.8 (797) · € 25.50 · En Stock
Click here:point_up_2:to get an answer to your question :writing_hand:explain the structure of graphite
Click here👆to get an answer to your question ✍️ Explain the structure of Graphite-
In Graphite each carbon atoms is united with three surrounding carbon atom through covalent bond and forms a sheet-like structure- These sheets or layers are stacked one above the other to form three dimensional structure-Each layer is made up of hexagons there is no covalent bonding between the layers- These layers are held together by weak Vander Wall-s physical forces- Hence these layers can slide over one another

The ABAB type of graphite. Figure 3. The ABC type of graphite
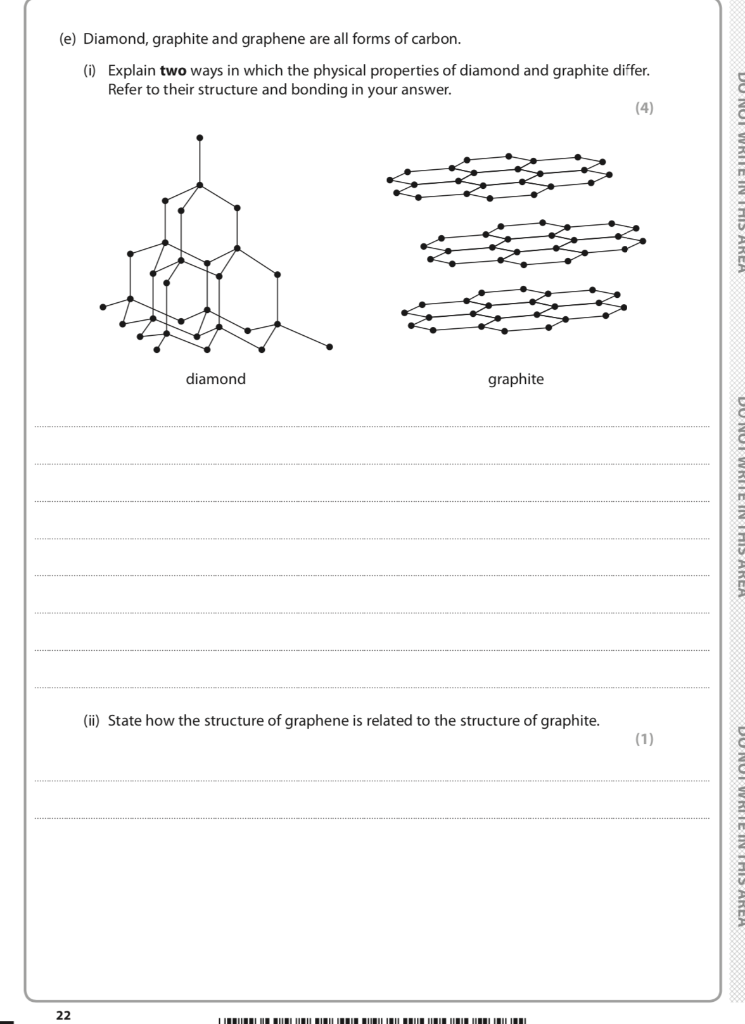
Solved (e) Diamond, graphite and graphene are all forms of

Rhombohedral Graphite - an overview

What is the Structure and Properties of Graphite

Covalent Diamond–Graphite Bonding: Mechanism of Catalytic Transformation

Graphite, Properties, Uses, Structure

Give reason : Graphite is used as a lubricant?
14.4A: Graphite and Diamond - Structure and Properties - Chemistry LibreTexts

Graphite and Its Structure - Diamond Films - Texas Powerful Smart

Graphite, Properties, Uses, Structure
What are the four ways in which the structure of graphite is different from the structures of diamonds? - Quora
What is the geometric structure of graphite, diamond, and buckyballs? - Quora
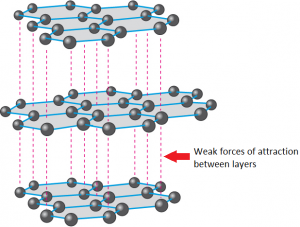
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry
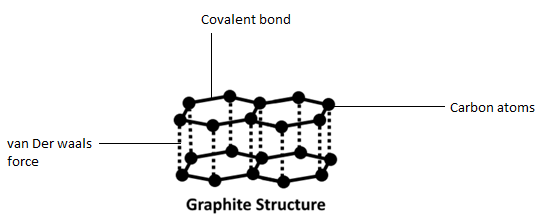
a) What is graphite? What substance is graphite made?(b) Describe the structure of graphite with the help of a labeled diagram.(c) Why is graphite a good conductor of electricity but diamond is
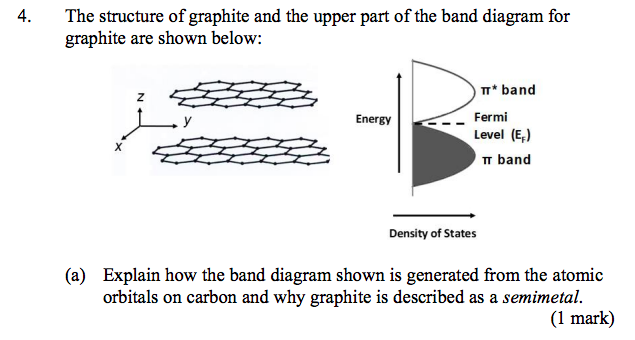
Solved 4. The structure of graphite and the upper part of












