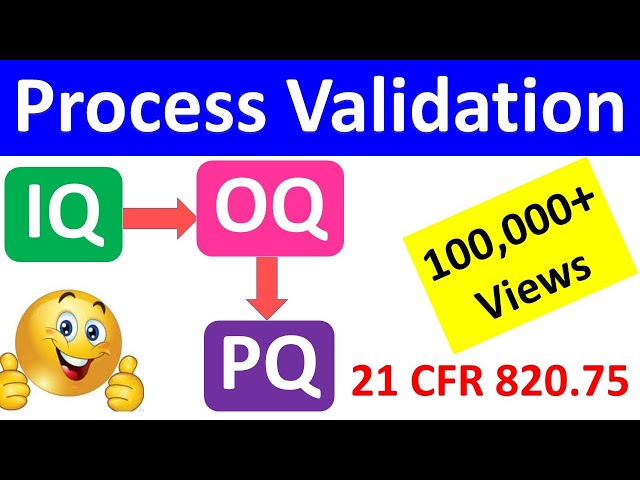
IQ OQ PQ, Process Validation, Equipment Validation
4.8 (511) · € 27.00 · En Stock
IQ OQ PQ are 3 pillars of Process Validation. IQ stands for Installation Qualification. OQ is Operational Qualification and PQ is Performance Qualification.
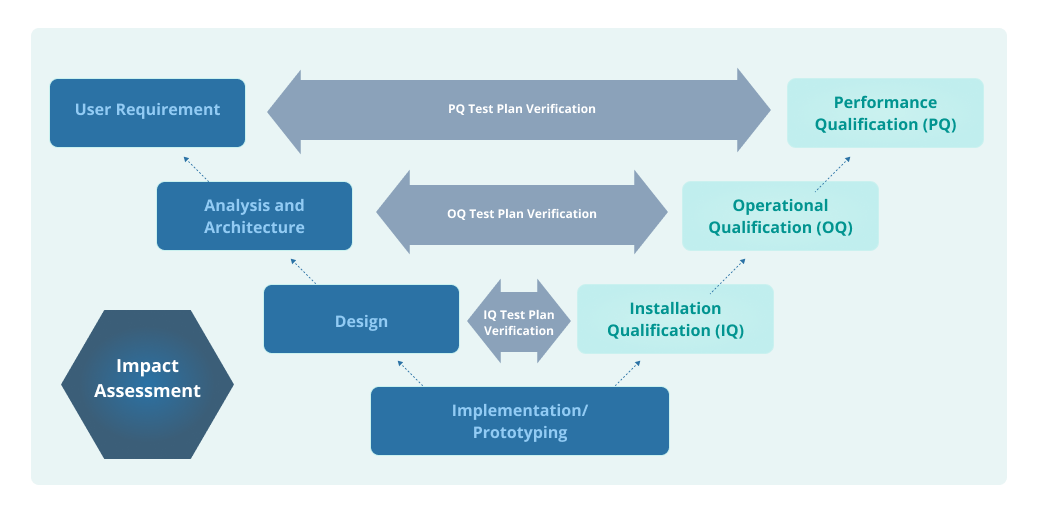
A Guide to IQ, OQ, and PQ in FDA-Regulated Industries

IQ OQ PQ Validation and Qualification Services
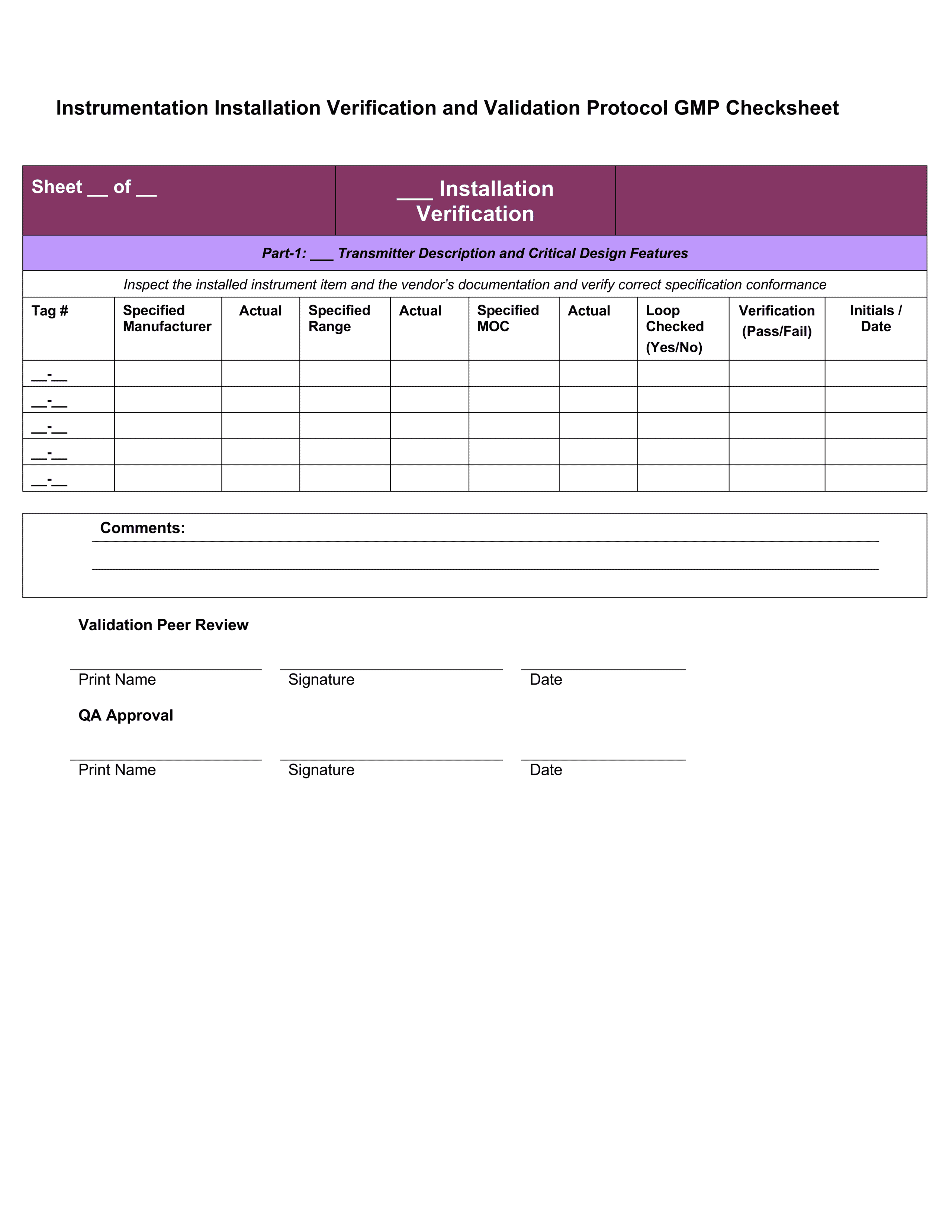
IQ OQ PQ Templates - Download 4 Professional Templates

Process Validation for Medical Devices, IQ OQ PQ Certification
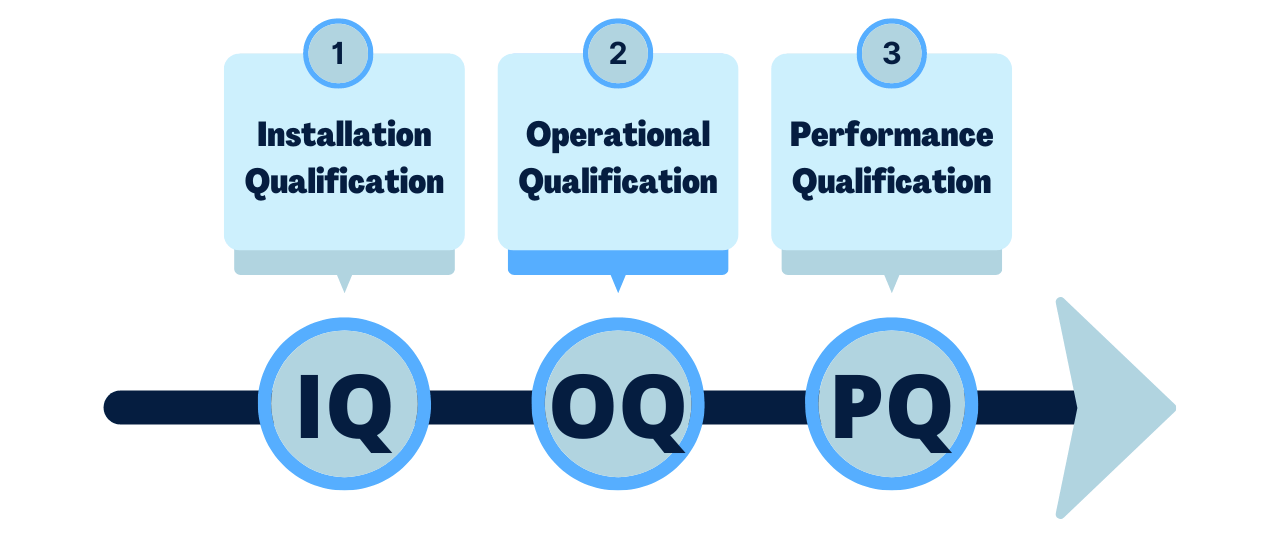
Fast Track ISO 13485 Process Validation Explained for your Medical Device
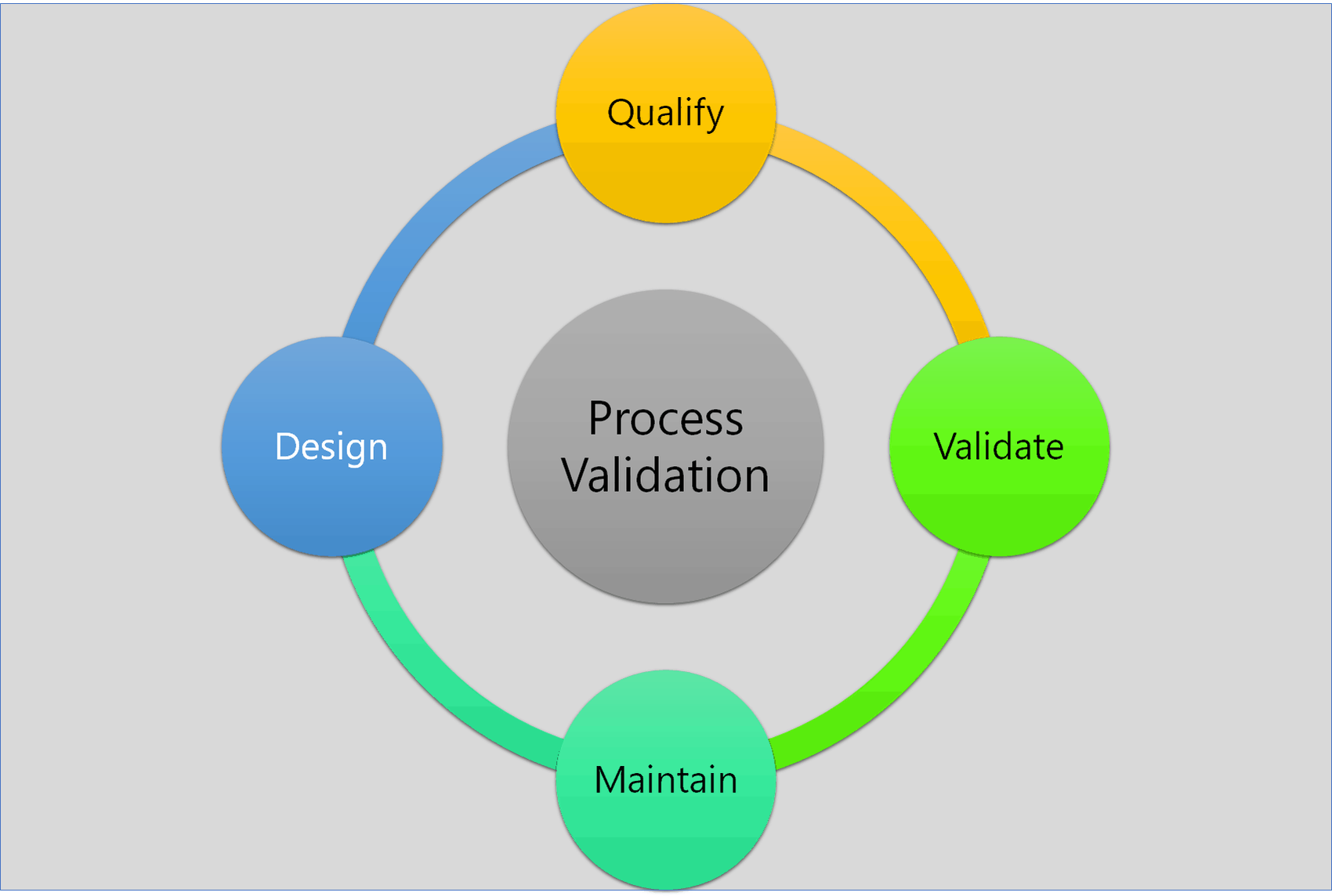
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP
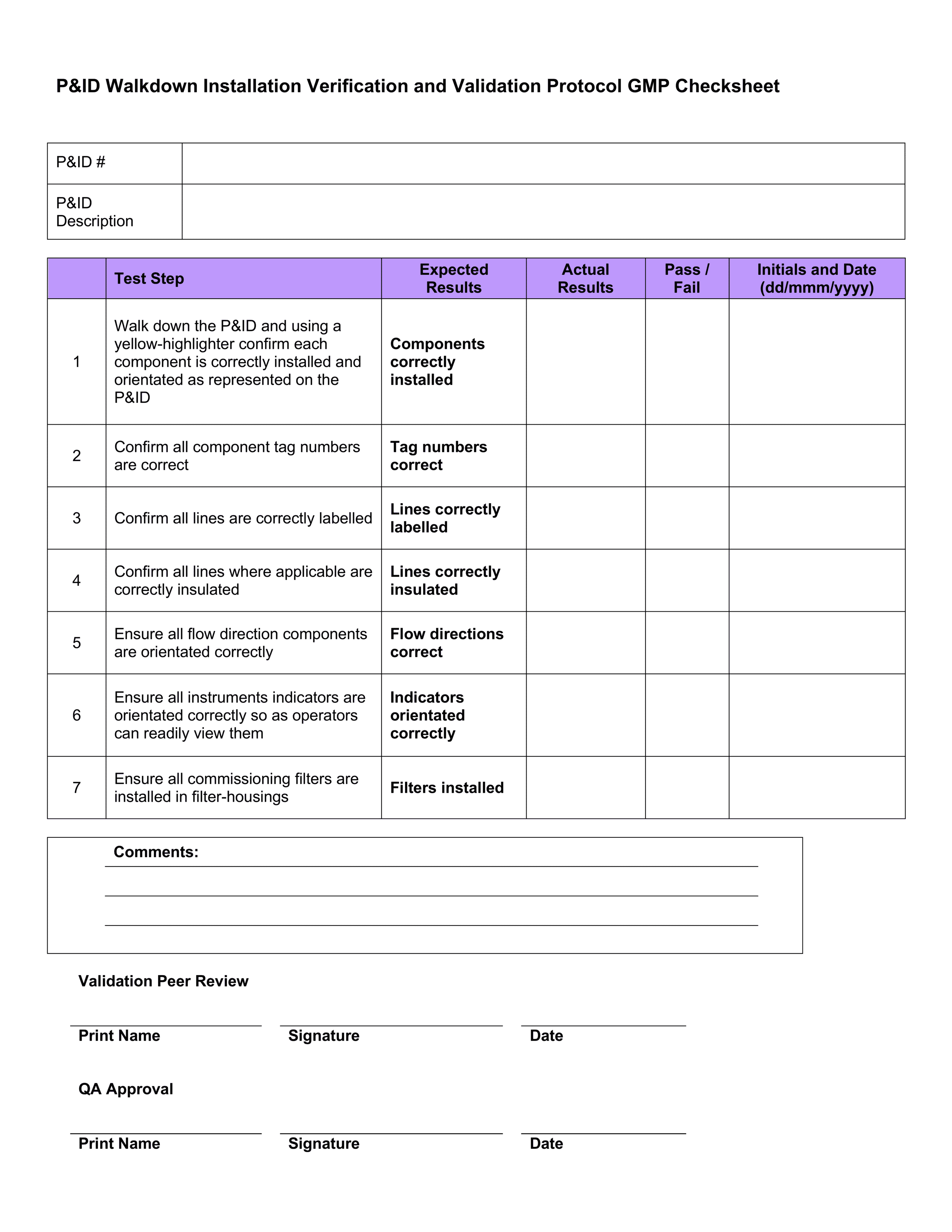
IQ OQ PQ Templates - Download 4 Professional Templates
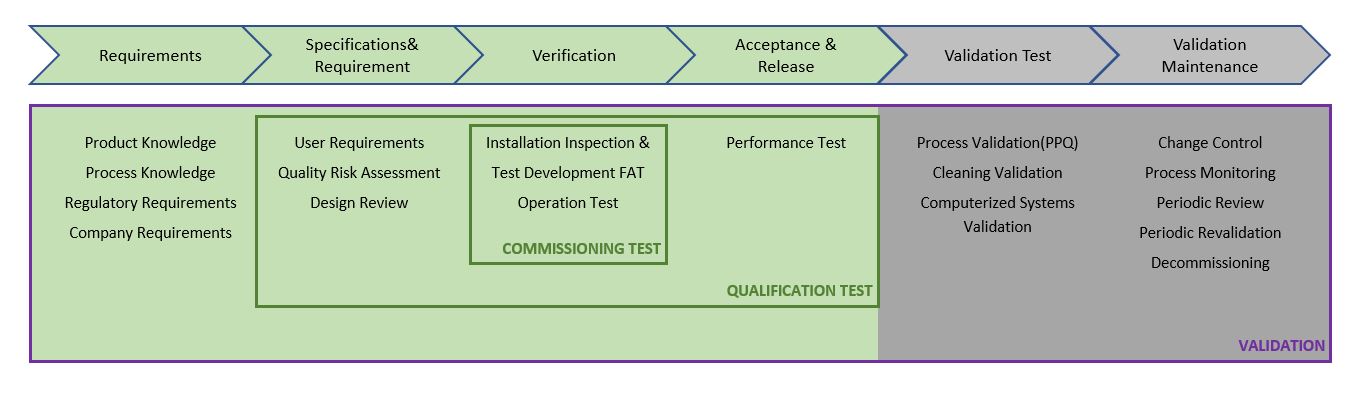
IQ, OQ, and PQ in the Pharmaceutical Industry – No deviation
Why did FDA change their Guideline on Process Validation? - ECA Academy

Why IQ/OQ/PQ Matters to You

What Are IQ OQ PQ, The 3 Q's Of Software Validation Process
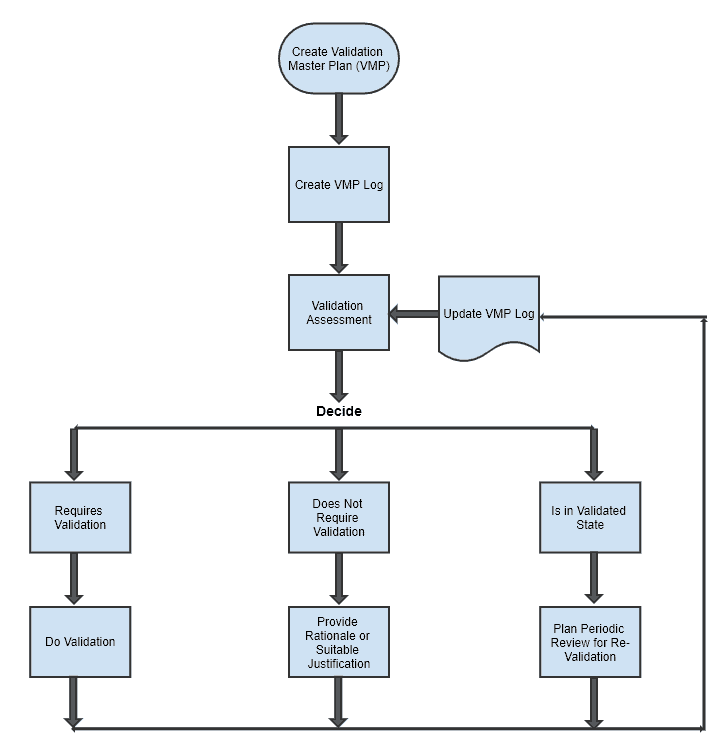
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP


)